Argon, refrigerated liquid (cryogenic liquid) appears as a colorless noncombustible liquid.Heavier than air. Contact may cause frostbite. May cause asphyxiation by displacement of air. Prolonged exposure to fire or intense heat may cause the container to rupture violently and rocket. Because argon has three stable isotopes that contribute to the element's atomic mass. An element's atomic mass is best thought of as the weighted average of the atomic masses of its stable isotopes. As you know, the identity of a chemical element depends exclusively on the number of protons an atom has in its nucleus - this is known as atomic number. In this case, you know for a fact that any.

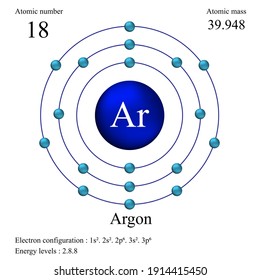
Why is argon placed before potassium in the modern periodic table?
1 Answer
Because the Periodic Table is based on atomic number,
Argon Atomic Mass Units
Explanation:

Argon Atom Mass In Kg
For potassium metal,

Argon Atomic Mass Rounded
Related questions
